H2O aq H2O l H3O aq OH- aq Hrxn 0 When the temperature of a sample of pure water is raised above 25 C A the hydronium ion concentration will be greater than the hydroxide ion. 100 6 ratings The strongest base is piperdine.
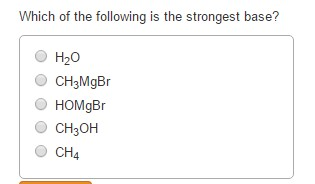
Solved Which Of The Following Is The Strongest Base H20 Chegg Com
View the full answer.
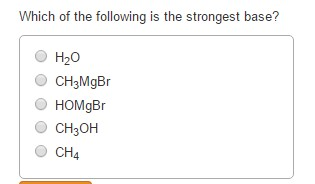
. Conjugate bases act in a similar fashion on strong bases. Here is the conjugate base and is the conjugate acid. Rank the bases from strongest to weakest.
Experts are tested by Chegg as specialists in their subject area. Aiodide anion I bfluoride anion F cbromide anion Br dchloride anion Cl. We review their content and use your feedback to keep the quality high.
1621 c Label if the following is a strong base weak base or species with negligible basicty. We review their content and use your feedback to keep the quality high. For example HF is a weak acid and when it will dissociate then it will give hydrogen ion and fluroide ion.
In compound D the nitrogen is not involved in resonance. ANaOH bNaCO3 cH2O dCH3OH. C the value of K w will increase.
Which of the following compounds cannot be a BrønstedLowry base. Previous question Next question. So they all are less basic.
A strong base b a weak base c a strong acid d a weak acid e none of these ANS. Strong acid and weak acid bond forming a compound that is not acidic. Reset Help н CH3CHC CH3CH2C CH3CC H Strongest Weakest Submit Request Answer - Part 3 List the following in order from strongest base to weakest base.
Which of the following is the. Start studying Chapter 9. CH3C a C Strongest The correct ranking cannot be determined.
Sulfuric acid H 2SO 4 e. Experts are tested by Chegg as specialists in their subject area. C6H50 OBCH 2CH O CHCEC ODCH 3 CH 2.
The reaction will be as follows. Write the formula for the conjugate acid and indicate whether the conjugate acid is a strong acid weak acid or a species with negligible acidity. ANH2 bNH3 cCH3CHN dCH3CN.
Which of the following anions is the strongest base. Which of the following is a conjugate acidbase pair. Which of the following compounds has the highest.
Which one of the following is not a strong acid. The equilibrium constant for the reaction. The weak acid in the buffer is attracted to strong acids and surrounds them neutralizing them.
Dehydrogenation through Cooperative Base Metal Catalysis Solved. The weak acid neutralizes acids. The conjugate base neutralizes acids B.
Each of the following pairs contains one. Correct option is D In the compounds AB and C the amino group is directly attached to the benzene ring. Whereas if an acid is strong then it will lead to the formation of a weak conjugate base.
LiOH NaOH KOH RbOH CsOH CaOH₂ SrOH₂ and BaOH₂. In compound D due to the presence of methylene group between amino group and the benzene ring the resonance between the lone pair of electrons on N and the benzene ring is not possible. A Loss of a proton from a base forms its conjugate acid.
So nitrogen has a slightly positive charge. Me 2NH O D. The conjugate base neutralizes bases.
Whereas HCl is a strong acid and its will be a weak. B the hydronium ion concentration will be less than the hydroxide ion concentration. Solve any question of Amines with-.
Part A List the folowing in order from strongest base to weakest base. There are 8 strong bases. C Gain of a proton by an acid forms its conjugate base.
NH 4 b. Which of the following is the strongest base. C NH4NH 3 PAGE.
Learn vocabulary terms and more with flashcards games and other study tools. So the lone pair of nitrogen is involved in resonance. Nitric acid HNO 3 d.
Which of the following is the strongest base. A HClOCl-b H2SO4SO42-c NH4NH3 d H3OOH-e none of these ANS. B a weak base PAGE.
Which of the following is the strongest base. Ust the following in order from strongest base to weakest base. D Brønsted-Lowry acid-base reactions always result in the transfer of a proton from a.
Which of the following statements about Brønsted-Lowry acids and bases is true. Hence this lone pair can be readily donated and hence benzylamine is the strongest base among the given bases. B Loss of a proton from an acid forms its conjugate base.
Hydrochloric acid HCl b. Rank the bases from strongest to weakest. Rank the bases from strongest to weakest.
Meo O ECL QUESTION 8 The stronger the acid the higher the pka and the lower the ka O True O False QUESTION 9 Which of the following is the strongest base. Carbonic acid H 2CO 3 16. So the lone pairs are not shared.
Perchloric acid HClO 4 c. Chemistry questions and answers. Which Of The Following Acids Is The Strongest Base.
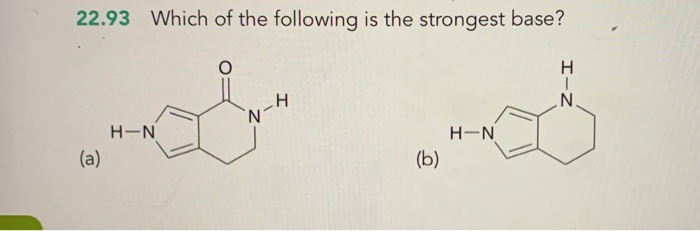
Solved 22 93 Which Of The Following Is The Strongest Base Chegg Com

Solved Which Of The Following Is The Strongest Base O Chegg Com
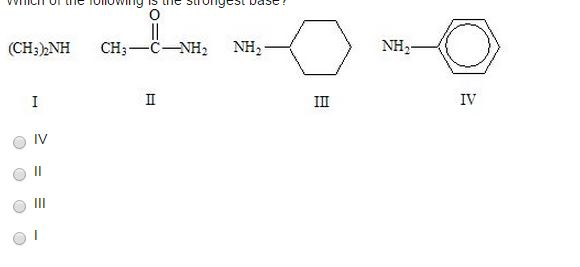
Solved Which Of The Following Is The Strongest Base Chegg Com
0 Comments